Watch on YouTube - Video notes - Video sections playlist - MES Science playlist
In this video I go over metabolism, which is the set of life-sustaining chemical reactions in organisms. Metabolic reactions can be categorized into catabolic (which break down molecules) or anabolic (which build up or synthesize molecules). Based on the first law of thermodynamics, energy is conversed, and second law, energy transfer leads to some loss of usable energy, thus chemical reactions require constant energy input to sustain themselves. In cells, energy can be transferred as electrons during redox reactions, which essentially means metabolic reactions are just slow motion combustion or burning reactions.
Timestamps:
Cells require energy to sustain cellular processes: 0:00
Work and energy in thermodynamics: 0:25
MES Note: Circular reasoning in definitions of work and energy: 1:09
Four laws of thermodynamics: 2:12
Classical thermodynamic processes: 5:08
Chemical reactions do not create new energy: 11:00
Redox (reduction-oxidation) reactions changes amount of elections of atoms: 12:07
Combustion is a redox reaction without distinct electron transfer as CO2 and H20 are formed: 18:01
Particulates and biological contaminants chart: 20:52
Exothermic reaction redox reactions of burning wood: 23:21
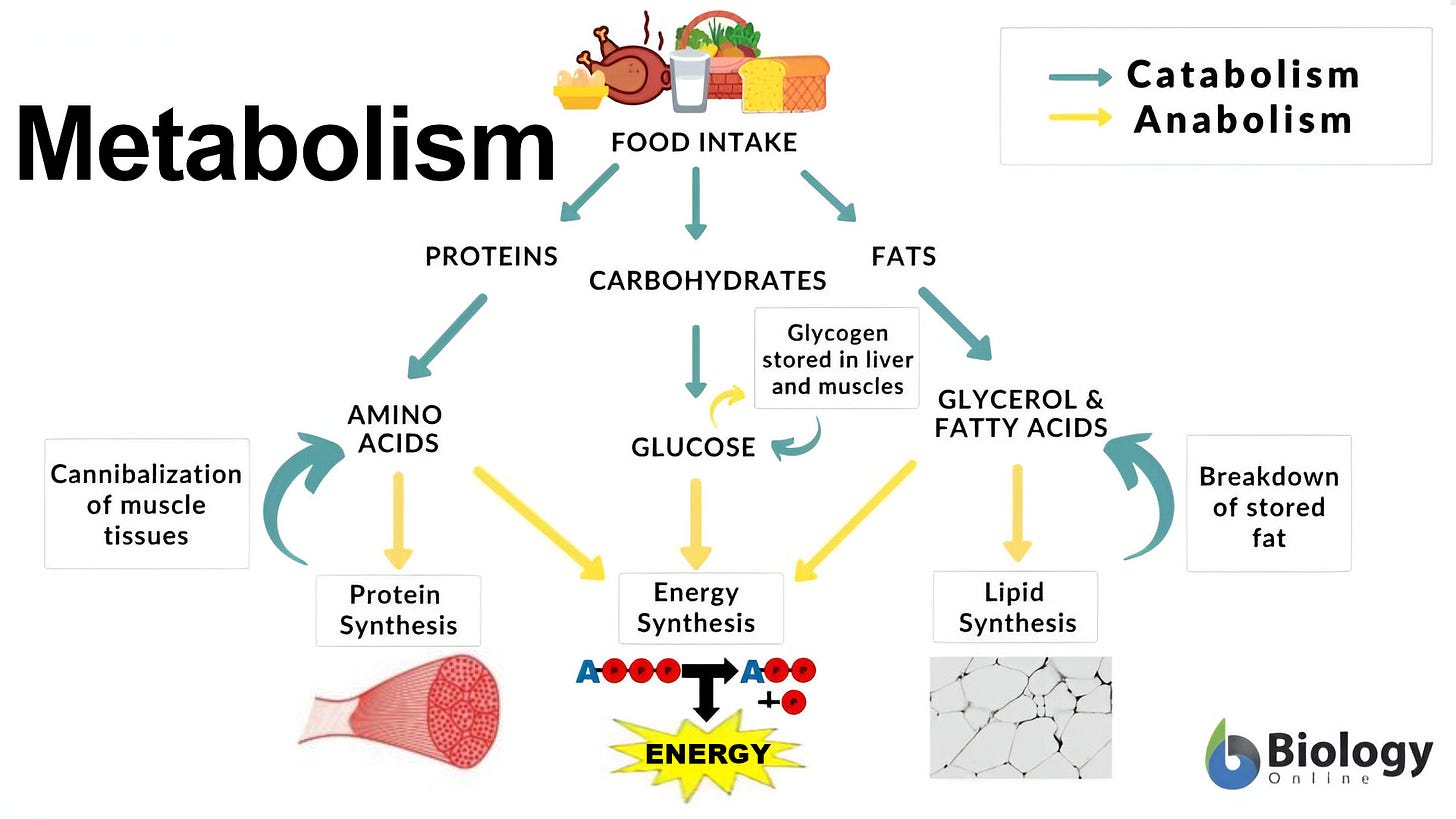
Metabolism are life-sustaining chemical reactions in organisms: 30:51
Metabolic chemical reactions are organized into metabolic pathways: 36:15
Fermentation is a metabolic process that changes organic substrates via enzymes: 37:54













Share this post