Watch on YouTube - Full video - Video notes - Video sections playlist - Sequences and Series playlist
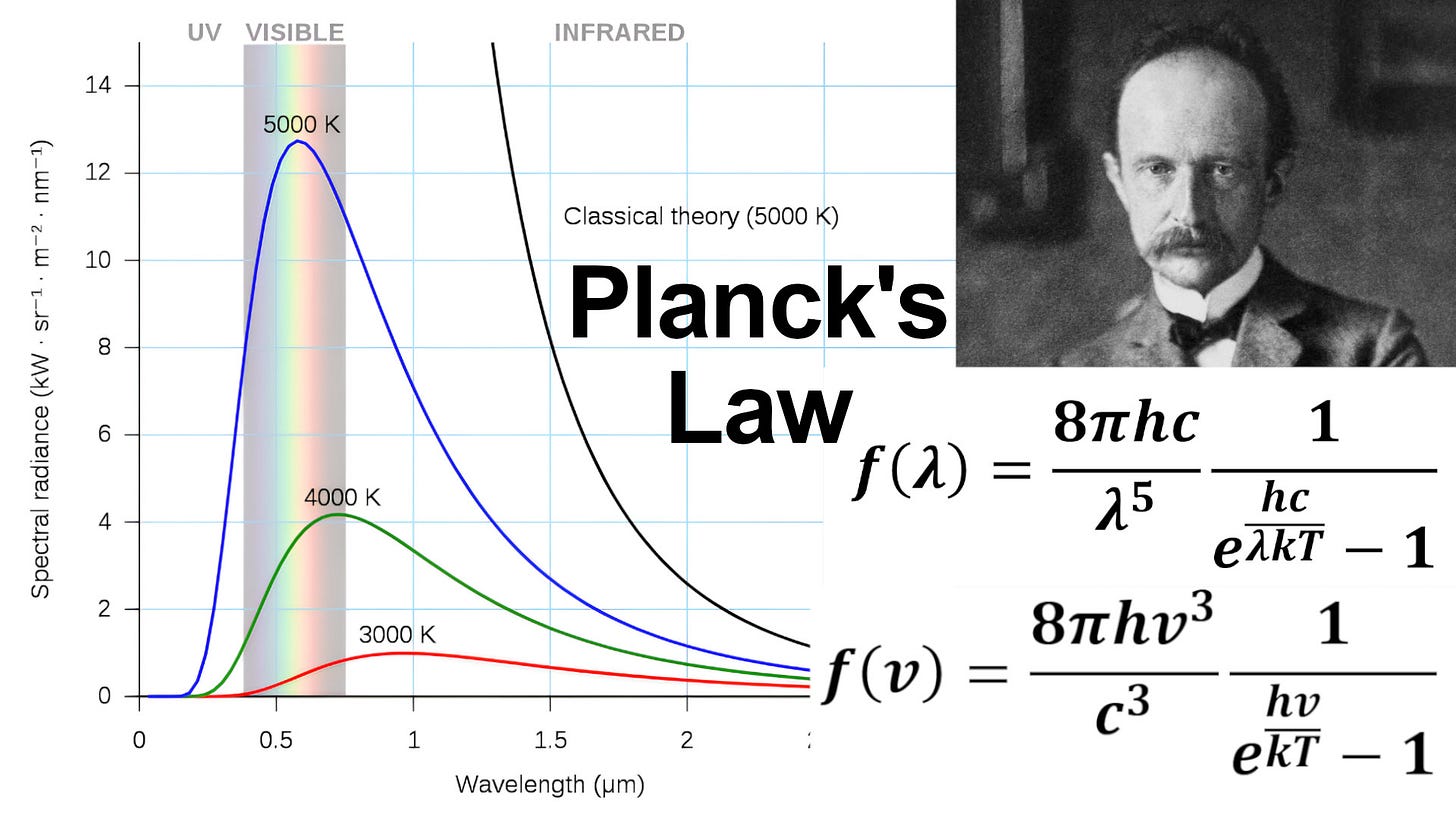
In this video, I provide a quick review of the history behind Planck's Law, the formula derived by Max Planck in 1900 to resolve the Ultraviolet Catastrophe for blackbody radiation. The classical theory for blackbody radiation, proposed by John William Strutt (3rd Baron Rayleigh) and James Jeans, predicted that the energy emitted by a blackbody would increase without bound as the wavelength decreased, leading to a divergence at short wavelengths. This was wildly inaccurate, as experiments showed the energy spectrum peaks at a certain wavelength and then decreases at both higher and lower wavelengths.
Through a process of trial and error, Max Planck derived a formula that matched experimental results by assuming that a hypothetical electrically charged oscillator in a cavity containing blackbody radiation can only emit energy in discrete quantities, or quanta. This was a significant departure from the previous theory of continuous energy intervals. Planck's revolutionary assumption of quantized energy emission pioneered the development of modern quantum mechanics.
Timestamps:
Planck's Law describes spectral density of electromagnetic radiation of a black body at thermal equilibrium: 0:00
1900, Max Planck proved that a hypothetical electrically charged oscillator can only change its energy in increments: 0:51
The Law: Spectral emissive power per unit area, per unit solid angle for particular radiation frequencies: 1:48
Increasing temperature increases total radiated energy and peaks at shorter wavelengths: 2:21
Planck's Law formula: 2:41
Graph of Comparison between Planck's Law vs Classical Theory (Rayleigh-Jeans Law): 3:34
Solid angle (units in steradians) is angle of projection for a specific area on the surface of a sphere: 4:16
Solid angle is used to quantify amount of radiation projected from a heat source: 4:49
Note that Wikipedia uses various versions of Planck's Law: 5:18 - Rayleigh-Jeans Law formula (classical theory): 5:34
Rayleigh-Jeans Law formula in terms of frequency: 6:22
Ultraviolet Catastrophe: Rayleigh Jeans-Law strongly disagrees at short wavelengths: 6:43
Planck's Law formula as energy density: 7:17
Planck's Law and Rayleigh-Jeans Law formulas (used in my Calculus book): 7:44
Planck's Law and Rayleigh-Jeans Law formulas as energy density in terms of frequency: 8:32













Share this post